Stephen H. Safe
Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, Texas, USA
http://ehpnet1.niehs.nih.gov/docs/2000/108p487-493safe/abstract.html
Address correspondence to S.H. Safe, Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, TX 77843-4466 USA. Telephone: (409) 845-5988. Fax: (409) 862-4929. E-mail: ssafe@cvm.tamu.eduThis work was supported by grants from the National Institutes of Health (ES04917 and ES09106) and the Texas Agricultural Experiment Station.
Received 13 September 1999; accepted 16 December 1999.
The effects of environmental endocrine disruptors on wildlife populations are being extensively investigated; adverse developmental and reproductive effects have been primarily linked to organochlorine compounds such as polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs), as well as alkylphenols derived from alkylphenol ethoxylate (AE) surfactants. Persistent organochlorine pollutants (POPs), including both pesticides such as DDT/DDE and PCBs, were among the first industrial compounds identified in the environment. Moreover, with improvement of analytical techniques, an ever-increasing number of structurally diverse POPs have been detected in environmental samples at low concentrations (2,6,13,14). The use and production of DDT and PCBs were restricted and banned in most countries in the 1970s; however, these compounds are still the most abundant POPs in most wildlife and human samples, even though their concentrations have significantly decreased over the past 30 years (14-16). Lower concentrations of POPs in the Great Lakes region are correlated with "dramatic improvements in reproductive success and significant increases in populations of cormorants, gulls, terns, herons and other predatory birds in the Great Lakes basin" (16).
There has been particular concern about the discharge and environmental persistence of AEs and their alkylphenol degradation products, which have been identified in relatively high concentrations in industrial sewage effluents and in sediments in lakes and rivers in Europe (4,17-21). Concentrations of these compounds in North American rivers and sediments tend to be lower. Soto et al. (20) showed that nonylphenols extracted from polystyrene were estrogenic, and Jobling and Sumpter (17) showed that the estrogen-regulated yolk protein, vitellogenin, was induced in male fish collected near sewage outflows in the United Kingdom. Moreover, Fairchild et al. (22) hypothesized that the estrogenic effects of nonylphenol, a solvent/emulsifier (an AE) used in pesticide spraying, may be related to declines of Atlantic salmon in Atlantic Canada. The widespread estrogenized fish populations in British rivers and estuaries have been extensively investigated (23-25) and were initially linked to nonylphenols and related compounds; however, the recent identification of etiologic agents from sewage treatment effluents that received mainly domestic wastes was somewhat surprising (23,24). The major estrogenic components were the natural hormones 17ß-estradiol (E2) and estrone, with minor amounts of the birth control pill ingredient 17-ethinylestradiol. Routledge et al. (23) concluded that
environmentally relevant concentrations of natural steroidal estrogens are sufficient to account for the levels of vitellogenin synthesis observed in caged male fish placed downstream of certain [sewage treatment works] effluent discharges in British rivers.
Thus, although initial concern regarding the estrogenic disruption of fish in U.K. rivers focused on synthetic estrogenic AE surfactants, human and possible animal discharges were the major sources of these environmental endocrine disruptors. This highlights the difficulties in assigning causality to environmental endocrine-disrupting chemicals without thoroughly investigating all potentially active agents.
Strong support for the endocrine-disruptor hypothesis has come from laboratory animal studies where increasing numbers of
synthetic chemicals have been shown to exhibit estrogenic/antiestrogenic, androgenic/antiandrogenic, and other endocrine-like activities (6,26-33). Included in this list of chemicals is p,p´-DDE, a major environmental contaminant that has been shown to exhibit antiandrogenic activity in both in vivo and in vitro models (28). Research from several laboratories has shown that in utero exposure to extremely low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD;
1 µg/kg), an aryl hydrocarbon receptor (AhR) agonist, can result in a host of neurodevelopmental and reproductive tract problems in juvenile and adult rodent offspring (26,27,30-32).
Studies on in utero exposure to the estrogenic drug diethylstilbestrol (DES) have served as an important model for delineating problems associated with exposure to estrogenic compounds in both animal models and in humans; DES-induced effects on the male and female reproductive tracts strongly support the endocrine-disruptor hypothesis (34,35). Bisphenol A, an important intermediate in the production of polycarbonates, is a weakly estrogenic industrial compound in most, but not all, assays (36-38). For example, bisphenol A induces mammary gland growth in Noble rats at doses as low as 0.1 mg/kg/day; this is similar to the dose required for the potent estrogen DES to induce the same response (34). The estrogenic activity of bisphenol A in CF-1 mice has also generated controversy (39-41); vom Saal et al. (39) reported that fetal exposure to low doses of bisphenol A (2 or 20 µg/kg/day) resulted in increased prostate weight in the male offspring. Results for bisphenol A, E2, and DES all gave low dose inverted U-shape dose-response curves for this effect; at higher doses, decreased prostate weight was observed in the offspring (39,40). In contrast, Cagen et al. (41) did not observe this low dose effect for bisphenol A or DES in CF-1 mice. Thus, the "low dose" hypothesis for this response should be resolved for bisphenol A and other estrogenic compounds.
Sperm counts. The concern over decreased sperm counts and male reproductive capacity was triggered by a paper on the meta-analysis of 61 sperm count studies which concluded that "There has been a genuine decline in semen quality over the past 50 years" (10). This study (10) and a subsequent paper (42) hypothesized that
We argue that the increasing incidence of reproductive abnormalities in the human male may be related to increased oestrogen exposure in utero.
PCBs and TCDD-like compounds, as well as DDE, may also contribute to this problem (42,43). The validity of the meta-analysis (10) was quickly debated (44-48), but more importantly, new research on this problem was initiated throughout the world. Results of single location and laboratory studies gave highly variable results. Some reports showed that over the last 15-25 years, there were significant decreases in sperm quantity, whereas other studies showed either no declines or slight increases (49-58). Auger et al. (49) investigated semen quality among fertile men in Paris and reported that
The mean concentration of sperm decreased by 2.1% per year from 86
106 per milliliter in 1973 to 60
106 per milliliter in 1992.
In contrast, using a similar approach, Bujan et al. (50) reported that from 1977 to 1992, "sperm concentration has not changed with time in the Toulouse area." In 1996, Fisch et al. (59) reported that semen quality of 1,283 men from three sperm banks in the United States had not declined over the last 25 years (1970-1995). The surprising results of this study were the large demographic differences in sperm counts: sperm donors from New York had the highest number (131.5 ± 3.5 106/mL, mean ± SEM), followed by Minnesota (100.8 ± 2.9
106/mL) and California (72.7 ± 3.1
106/mL).
In a separate study, Paulsen et al. (54) reported no change in sperm counts in Washington State between 1972 and 1993 (46.5 106/mL to 52
106/mL); by accounting for geographic differences in an analysis of 29 U.S. studies from 1938 to 1996, Saidi et al (60) reported "no significant change in sperm counts during the last 60 years." Geographic differences in sperm counts have been reported in French men (61) and Danish men (62) and also in a Canadian study among 11 academic fertility centers (63). The latter report shows that among 48,968 samples of sperm taken from Canadian men between 1984 and 1996, there was a significant overall downward trend in sperm counts (63); however, when the data from 1975 to 1983 were included, "there was no significant trend in sperm density" (63). The analysis of data from the individual centers (1984-1996) showed that 6 centers had a downward trend and 5 centers had small but insignificant increases in sperm counts. However, the most remarkable results from these studies were the differences (geographic) between centers: in 1984, sperm counts ranged from 51
106/mL to 121
106/mL, and in 1996, these values ranged from 48
106/mL to 137
106/mL (63). The predictive value of sperm count data taken from self-selected volunteers is clearly subject to multiple variables including measurement methods, temperatures, time of day, and seasonal variability. Handelsman (52) reported that mean sperm counts taken from five different sets of volunteers at the same hospital in Sydney, Australia, varied from 142
106/mL to 63
106/mL, which exceeded the differences in the decline in sperm counts reported in the 1992 meta-analysis by Carlsen et al. (10). Handelsman (52) concluded that
This highlights the invalidity of extrapolating similar findings on sperm counts of selected volunteers to the general male community or in using such study groups to characterize sperm counts in supposedly healthy men.
These data suggest that we do not know if sperm counts are actually up or down. Our knowledge of sperm counts and their temporal variability in normal populations is minimal, and the contributions of the environment (i.e., lifestyle, diet, contaminants, etc.) are also unknown.
Hypospadias and cryptorchidism. Cryptorchidism is a condition in which one or both testicles have not descended, and hypospadias occurs when the urethral opening is displaced. Both of these responses have been observed in male offspring of rodents exposed in utero to estrogenic and antiandrogenic compounds (64,65). Weidner et al. (66) reported a significantly increased risk of cryptorchidism, but not hypospadias, among male offspring of female (but not male) gardeners. This study suggests a possible link between in utero exposure to agricultural chemicals used in gardening; however, the identity of potential toxic chemicals and their mode of action are unknown.
Sharpe and Skakkebæk (42), suggested that decreased male reproductive capacity may be related to exposure to endocrine disruptors (42), and pointed out that some studies reported increases in hypospadias and cryptochidism in male infants. Paulozzi (67) analyzed international trends in rates of cryptorchidism and hypospadias from several different countries and categorized regions by gross domestic product (in 1984). Hypospadias increased in more affluent areas but not in less affluent areas up to 1985, whereas no increases have been observed since 1985. Before 1985, there were also region-specific trends in rates of cryptorchidism (increases in two U.S. and one South American system); however, since 1985, rates have actually declined in most systems (67). Thus, the specter of a global decrease in male reproductive capacity is not supported by international trends for hypospadias or cryptorchidism; however, like the sperm count data, there is some evidence that incidence of these problems is also dependent on demography.
Testicular cancer. Testicular cancer is one of the major types of cancer in young men, and there is evidence suggesting a prenatal etiology (68). Cryptorchidism is one of the known risk factors for testicular cancer, suggesting that in utero or early postnatal exposure to estrogens or antiandrogens may contribute to development of this tumor in young men (68-72). Testicular cancer is generally increasing in most countries, and it was suggested that p,p´-DDE, an antiandrogen, could play a role in the development of this cancer (43). However, Ekbom et al. (15) pointed out that DDE concentrations in breast milk in the four Scandinavian countries are not significantly different, whereas the incidence of testicular cancer (1985-1989) varies from 14.5/105 males in Denmark (highest) to 3.6/105 males in Finland (lowest). Moreover, as the overall incidence of testicular cancer has increased in all Scandinavian countries during the last 25-30 years, there has been an 80-90% decrease in average breast milk DDE concentrations, showing an inverse relationship between testicular cancer and DDE concentration (15). Similar inverse correlation can be observed in most other developed countries, including the United States (73). Many countries in similar regions (e.g., bordering the North Sea) do not have major differences in human or environmental concentrations of persistent organochlorine pollutants, and differences in production and human exposure to other synthetic chemicals are unlikely. Environmental exposures have not been linked or correlated with testicular cancer, and the environmental and lifestyle factors, including diet and occupational exposures, that are responsible for this disease are unknown and should be investigated.
Sex ratios and endocrine disruptors. A recent study (74) reported that accidental exposure to high concentrations of TCDD in Seveso resulted in lower sex ratios (male/female) at birth; this observation has been noted for high level occupational exposures to some chemicals (74-76). There is evidence from several countries, including a recent U.S. study, that in the past few decades there have been small but significant decreases in sex ratios (77-79). The potential role of male/female sex ratios as sentinel health indicators "that may be linked to environmental factors" was proposed by Davis et al. (80), based on their observations that the proportion of males born in Denmark (1950-1994), The Netherlands (1950-1994), Canada (1970-1990), and the United States (1970- 1990) had decreased. However, in the United States, although the overall sex ratio decreased from 1.053 in 1969 to 1.049 in 1995, this small decline was observed only for Cau-casians but not African Americans (77); the authors concluded that "environmental exposures are unlikely to account for the observed trends." In a more recent comprehensive study, Vartainen et al. (81) examined sex ratios in Finland over the past 250 years. From 1751 to 1920, there was an increase in male/female birth ratios, and with the exception of pre- and post-World War I and II years, there has been a decline in these ratios since 1920. The authors concluded that
the present data do not support the hypothesis that agricultural or industrial environmental estrogens play any significant role
because, in Finland, major production and exposures to these types of chemicals occurred after 1950. James (82) initially proposed that newborn sex ratios may be a useful indicator of male reproductive hazard but has subsequently concluded that "population sex ratios at birth are not useful monitors of reproductive hazard."
Fertility. Temporal changes in human fertility have not been extensively investigated; however, a recent study in Sweden (83) concluded that
a decrease in male fertility cannot be ruled out ... but if present, it is minor and totally outweighed by other favorable developments.
Akre et al. (83) observed declining infertility among successive birth cohorts, and "this strongly argues against a substantial deterioration in sperm quality." This was explained by the decreasing incidence of gonorrhea between 1970 and 1992. Previous studies that demonstrated adverse effects of DES on male and female offspring are unquestionable (34,35); however, Wilcox et al. (84) reported that fertility of DES-exposed males was not significantly different from males in a control group. DES was not used in Finland, but in a recent study, Hemminki et al. (85) reported reproductive effects of in utero exposure to estrogen and progestin drugs on male and female offspring. There were some differences in times of birth between controls and hormone- or drug-exposed individuals (e.g., time to first birth after marriage, mean time between first and second live birth); however, the overall fertility in both groups was similar. Hemminki et al. (85) concluded that
Estrogen- and progestin-containing drugs as used in the study population did not have much impact on the fertility of offspring.
Thus, pharmacologic doses of estrogenic drugs did not affect fertility, and it is unlikely that much lower concentrations of weakly estrogenic industrial compounds would affect fertility. Thus, with the exception of testicular cancer, data from more recent studies suggest that there does not appear to be a worldwide decrease in disorders of the male reproductive tract.
In 1992-1993, two studies from Connecticut and New York reported higher concentrations of PCBs and DDE, respectively, in breast cancer patients as compared to controls (86,87); this generated the hypothesis that xenoestrogens, such as PCBs and DDE, were preventable causes of breast cancer (88). This hypothesis was supported by the two studies on breast cancer patients (86,87) and also by in vitro metabolic data on chemical-induced metabolism of E2 to 2-hydroxyestrone (2-OHE1) and 16-hydroxyestrone (16
-OHE1) metabolites in MCF-7 human breast cancer cells (89). The hypothesis of Davis et al. (88) was criticized on several counts (11), and new research on the role of organochlorine contaminants in the incidence of breast cancer has clarified some of these issues.
PCB and DDE concentrations in breast cancer patients. I was particularly skeptical about the biologic plausibility of the xenoestrogen hypothesis because occupational exposures to high concentrations of PCBs or DDE have not been associated with increased risk for breast cancer (11). In rodent carcinogen-induced mammary cancer models, DDE and 3,3´,4,4´-tetrachlorobiphenyl both increased and decreased mammary tumor formation and growth (90-93), and the increase in mammary tumor formation was primarily associated with altered carcinogen metabolism (91,92). Both TCDD and higher chlorinated PCB mixtures inhibited formation of spontaneous mammary tumors in 2-year-old female Sprague-Dawley rats (33,94); this was consistent with the antiestrogenic activity associated with AhR agonists in breast cancer (95,96).
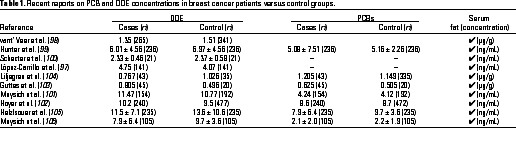
Several recent studies on DDE and PCB concentrations in breast cancer patients versus controls have been carried out in Europe, Asia, North and South America, and most studies indicate that levels of these organochlorine contaminants are not significantly different in the two groups (Table 1) (97-106). These data are consistent with an earlier report by Krieger et al. (107) in a California cohort. Several studies have also investigated risk for breast cancer and correlations with other organochlorine compounds or other parameters. For example, Moyisch et al. (101,106) reported an odds ratio (OR) of 1.8 for women (parous women who had never lactated) with high concentrations of hexachlorobenzene (HCB), but Zheng et al. (108) did not observe higher concentrations of HCB in patients in Connecticut (304 cases and 186 controls) and concluded that
our study does not support a positive association between environmental exposure to HCB and risk of breast cancer.
In a study in Copenhagen, Hoyer et al. (102) reported that although correlations for PCBs were not observed, the pesticide dieldrin was associated with an increased risk for breast cancer. The authors concluded that
these findings support the hypothesis that exposure to xenoestrogens may increase the risk of breast cancer.
Dieldrin is an exceedingly weak estrogen in most assays, and the biologic plausibility that trace concentrations of this contaminant play a role in breast cancer is minimal. Some recent studies have combined differences in DDE or PCB concentrations with drug-metabolizing enzyme polymorphisms in breast cancer patients and control groups (mixtures and congeners) to further investigate correlations with breast cancer (106); this approach in future studies may lead to new insights.
Estrogen metabolite ratios. Bradlow et al. (89) investigated the effects of various chemicals, including several organochlorine pesticides, on the metabolism of E2 to 2-OHE1 and 16-OHE1 in MCF-7 human breast cancer cells and concluded that "the ratio of 16
-OHE1/2-OHE1 may provide a marker for the risk of breast cancer." PCBs, DDE, and other weakly estrogenic pesticides induced higher 16
-OHE1/2-OHE1 ratios, and these data were initially used to support the xenoestrogen hypothesis in early reports showing higher concentrations of PCBs or DDE in breast cancer patients versus controls. The predictive utility of the ratio was based on reports that 16
-OHE1 was highly estrogenic and higher levels of this metabolite were observed in a small cohort of breast cancer patients (89,109,110). In contrast, 2-OHE1 exhibits partial antiestrogenic activity and was labeled by Bradlow et al. as "the 'good' estrogen" (110). In my laboratory, we used the radiometric assay, as previously described (89), to investigate the effects of a diverse spectrum of estrogens, antiestrogens, and mammary carcinogens on estrogen metabolism (111,112). The results showed that the metabolite ratios in our study were not predictive of carcinogens, estrogens, or antiestrogens, and metabolite ratios varied with the concentration of some compounds in this assay. For example, the triphenylethylene antiestrogen 4´-hydroxytamoxifen decreased the ratio, whereas the potent steroidal antiestrogen ICI 182,780 increased the ratio; we also observed other inconsistencies between compounds with similar effects on mammary cancer (111,112). We concluded that induction of this metabolite ratio in MCF-7 breast cancer cells was not predictive for a wide variety of chemicals that may affect breast cancer in vivo (112). Some additional studies question the use of the urinary 16
-OHE1/2-OHE1 ratio as a predictor of risk for breast cancer (113-115). In a recent study, Ursin et al. (115) reported that in 66 postmenopausal breast cancer patients and 76 control subjects,
the ratio of 2-OHE1 to 16a-OHE1 was 1.1% higher in the patients (p = 0.84) contrary to the hypothesis.
The authors concluded that
this study does not support the hypothesis that the ratio of the two hydroxylated metabolites (2-OHE1/16
-OHE1) is an important risk factor for breast cancer.
The potential adverse role of endocrine disruptors in the diet during critical periods of development will depend not only on intake concentrations of these compounds but also on their concentrations in serum and in the fetus. Intake concentrations of synthetic estrogenic compounds and AhR agonists are low as compared to dietary intakes of natural phytoestrogens and AhR-active compounds (e.g., indole-3-carbinol) in fruits and vegetables (11). In one collaborative study, we compared the estrogenic potency of one glass of red wine (200 mL) with the corresponding potency of the estimated daily intake of organochlorine pesticide residues in food (116) (Figure 1). The reconstituted mixture in this study included the following weakly estrogenic organochlorine contaminants: 1,1,1-trichloro-2-(p-chlorophenyl)-2-(o-chlorophenyl)ethane, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene, endosulfan-1, endosulfan-2, p,p´-methoxychlor, and toxaphene. The relative proportion of each chemical in the mixture resembled the composition reported in a recent U.S. Food and Drug Administration market basket survey (117). Using a series of seven in vitro assays, the calculated estrogen equivalents (EQs) in extracts from 200 mL red cabernet wine varied from 0.15 to 3.68 µg/day. In contrast, the EQs for consumption of organochlorine pesticides (2.44 µg/day) varied from nondetectable to 1.24 ng/day. These data, coupled with EQs for other foods (118), demonstrate that dietary intakes of naturally occurring phytoestrogens far exceed intakes of organochlorine xenoestrogens. However, these data should not be overinterpreted because organochlorine compounds bioaccumulate and can be identified in serum and because dietary intake levels of other xenoestrogens are unknown.
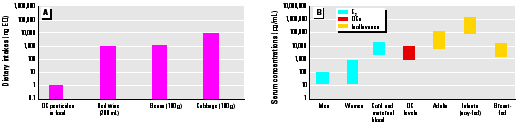
Figure 1. (A) Estrogen equivalents (EQs) in various food products and organochlorine compounds (OCs) (117) and (B) range of human serum concentrations of natural estrogens, isoflavones, and OCs (116,119) in different groups. EQs (ng) were 0-1 for OC pesticides in food, 200-3,000 for red wine, 200-1,000 for beans, and 24,000 for cabbage.
Recent reports have investigated serum concentrations of some phytoestrogens (119,120), and these can be compared to endogenous hormone and organochlorine xenoestrogen serum concentrations in human populations. A recent study on women in Long Island, New York, showed that total serum concentrations of organochlorine pesticides plus PCBs were < 10 ng/mL (121); these values correspond to serum concentrations from women sampled within the last 5-10 years (Table 1). Setchell et al. (119) recently summarized serum concentrations of endogenous estrogenic hormones in men, women, and children as well as concentrations of estrogenic isoflavones in various groups. Serum concentrations of estradiol in men, women, and neonates are variable (between 10 and 500 pg/mL); however, concentrations in maternal blood and cord blood are similar to concentrations of organochlorine compounds (10,000 pg/mL). On the basis of the low estrogenic potencies of these xenoestrogens, it is unlikely that their effects as estrogens are important. This does not exclude endocrine- and estrogen-independent toxic actions of organochlorine compounds that are currently being investigated in several laboratories. Serum concentrations of estrogenic isoflavones (primarily genistein, daidzein, and equol) can range from 552,000 to 1,755,000 pg/mL (mean = 988,000 pg/mL) in soy-fed infants; these are approximately 100-fold higher than organochlorine concentrations (119). High isoflavone concentrations (~ 100,000 pg/mL) have also been detected in Japanese men (119,120). Thus, on the basis of current analytical data, soy-fed infants are the group with the highest exposure to estrogenic compounds during critical periods of development. Most studies associate consumption of phytoestrogen-containing foods with health benefits (122,123); however, as reported by Setchell et al. (119),
To allay increasing concerns about soy-based formulas, long-term follow-up studies are needed to assess the potential beneficial or adverse effects of phyto-oestrogen exposure in early life.
Environmental concentrations of persistent organochlorine compounds have been decreasing over the past two decades, and this correlates with remarkable advances in the detection of exceedingly low levels of these compounds in human populations. Undoubtedly, correlational studies of human diseases with tissue and serum concentrations of organochlorine compounds will continue, and due to the large number of these compounds, positive correlations with some diseases will undoubtedly be made. It is important that interpretation of data obtained from these studies consider biologic plausibility and temporal trends in concentrations as well as additional correlative results from other reports.
Results of recent studies suggest that there is not a global decrease in male reproductive capacity and that an etiologic role for xenoestrogens in female breast cancer is unlikely. It is possible that new scientific evidence may reinforce or weaken these conclusions; it is also important to carefully validate and replicate findings before media announcements that may contribute to unnecessary fear and worry by the public. A recent book, The Culture of Fear (124), has addressed some of the issues regarding unreasonable fears by the public; it states,
We compound our worries beyond all reason. Life expectancy in the United States has doubled during the twentieth century. We are better able to cure and control diseases than any other civilization in history. Yet, we hear that phenomenal numbers of us are dreadfully ill.
The role of endocrine disruptors and human disease has not been fully resolved; however, at present the evidence is not compelling.
Note: A recent paper by many of the same coauthors of the sperm count meta-analysis study (10) have now reported "a significant increase in mean sperm concentration from 53.0 106/mL in 1977 to 72.7
106/mL in 1995" among donors to the Central Sperm Bank in Copenhagen (125).
References and Notes
1. Colborn T, Vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378-384 (1993).
2. Giesy JP, Ludwig JP, Tillitt DE. Deformities of birds in the Great Lakes region: assigning causality. Environ Sci Technol 28:128A-135A (1994).
3. Fry DM. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect 103(suppl 7):165-171 (1995).
4. Sumpter JP, Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect 103(suppl 7):173-178 (1995).
5. Guillette LJ Jr, Crain DA, Rooney AA, Pickford DB. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect 103(suppl 7):157-164 (1995).
6. Birnbaum LS. Developmental effects of dioxin. Environ Health Perspect 103:89-94 (1995).
7. de Solla SR, Bishop CA, Van der Kraak G, Brooks RJ. Impact of organochlorine contamination on levels of sex hormones and external morphology of common snapping turtles (Chelydra serpentina serpentina) in Ontario, Canada. Environ Health Perspect 106:253-260 (1998).
8. Guillette LJ Jr, Pickford DB, Crain DA, Rooney AA, Percival HF. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol 101:32-42 (1996).
9. Guillette LJ Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect 102:680-688 (1994).
10. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for the decreasing quality of semen during the past 50 years. Br Med J 305:609-612 (1992).
11. Safe S. Environmental and dietary estrogens and human health: is there a problem? Environ Health Perspect 103:346-351 (1995).
12. National Research Council. Hormonally Active Agents in the Environment. Washington, DC:National Academy Press, 1999.
13. Vallack HW, Bakker DJ, Brandt I, Broström-Lundén E, Brouwer A, Bull KR, Gough C, Guardans R, Holoubek I, Jansson B, et al. Controlling persistent organic pollutants--what next? Environ Toxicol Pharmacol 6:143-175 (1998).
14. Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol 120:1-82 (1991).
15. Ekbom A, Wicklund-Glynn A, Adami HO. DDT and testicular cancer. Nature 347:553-554 (1996).
16. Tremblay NW, Gilman AP. Human health, the Great Lakes, and environmental pollution: a 1994 perspective. Environ Health Perspect 103(suppl 9):3-5 (1995).
17. Jobling S, Sumpter JP. Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic Toxicol 27:361-372 (1993).
18. Nimrod AC, Benson WH. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol 26:335-364 (1996).
19. Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem 15:194-202 (1996).
20. Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonylphenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect 92:167-173 (1991).
21. Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science 225:623-625 (1984).
22. Fairchild WL, Swansburg EO, Arsenault JT, Brown SB. Does an association between pesticide use and subsequent declines in catch of Atlantic salmon (Salmo salar) represent a case of endocrine disruption? Environ Health Perspect 107:349-357 (1999).
23. Routledge EJ, Sheahan D, Desbrow C, Brighty GC, Waldock M, Sumpter JP. Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ Sci Technol 32:1559-1565 (1998).
24. Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol 32:1549-1558 (1998).
25. Allen Y, Scott AP, Matthiessen P, Haworth S, Thain JE, Feist S. Survey of estrogenic activity in United Kingdom estuarine and coastal waters and its effects on gonadal development of the flounder Platichthys flesus. Environ Toxicol Chem 18:1791-1800 (1999).
26. Gray LE Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol Appl Pharmacol 131:108-118 (1995).
27. Gray LE Jr, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol Appl Pharmacol 133:285-294 (1987).
28. Kelce WR, Stone CR, Laws SC, Gray LE. Persistent DDT metabolite p,p´-DDE is a potent androgen receptor antagonist. Nature 375:581-586 (1995).
29. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van der Saag PT, Van der Burg B, Gustafsson J-Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor ß. Endocrinology 139:4252-4263 (1998).
30. Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactional exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 3. Effects of spermatogenesis and reproductive capability. Toxicol Appl Pharmacol 114:118-126 (1992).
31. Mably TA, Moore RW, Goy RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 2. Effects on sexual behavior and the regulation of luteinizing hormone secretion in adulthood. Toxicol Appl Pharmacol 114:108-117 (1992).
32. Mably TA, Moore RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Effects on androgenic status. Toxicol Appl Pharmacol 114:97-107 (1992).
33. Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, et al. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol Sci 41:62-76 (1998).
34. Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril 72:1-7 (1999).
35. Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect 103(suppl 7):83-87 (1995).
36. Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132:2279-2286 (1993).
37. Colerangle JB, Roy D. Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J Steroid Biochem Mol Biol 60:153-160 (1997).
38. Gould JC, Leonard LS, Maness SC, Wagner BL, Connor K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor
39. vom Saal FS, Timms BG, Montano MM, Thayer KA, Nagel SC, Dhar MG, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA 94:2056-2061 (1997).
40. Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect 105:70-76 (1997).
41. Cagen SZ, Waechter JM Jr, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. Normal reproductive organ development in CF-1 mice following prenatal exposure to bisphenol A. Toxicol Sci 50:36-44 (1999).
42. Sharpe RM, Skakkebaek NF. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract. Lancet 341:1392-1395 (1993).
43. Sharpe RM. Reproductive biology. Another DDT connection. Nature 375:538-539 (1995).
44. Olsen GW, Bodner KM, Ramlow JM, Ross CE, Lipshultz LI. Have sperm counts been reduced 50 percent in 50 years? A statistical model revisited. Fertil Steril 63:887-893 (1995).
45. Becker S, Berhane K. A meta-analysis of 61 sperm count studies revisited. Fertil Steril 67:1103-1108 (1997).
46. Fisch H, Goluboff ET. Geographic variations in sperm counts: a potential cause of bias in studies of semen quality. Fertil Steril 65:1044-1046 (1996).
47. Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect 105:1228-1232 (1998).
48. Lerchl A, Nieschlag E. Decreasing sperm counts? A critical (re)view. Exp Clin Endocrinol Diabetes 104:301-307 (1996).
49. Auger J, Kuntsmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 332:281-285 (1995).
50. Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. Br Med J 312:471-472 (1996).
51. Irvine S, Cawood E, Richardson D, MacDonald E, Aitken J. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. Br Med J 312:467-471 (1996).
52. Handelsman DJ. Sperm output of healthy men in Australia: magnitude of bias due to self-selected volunteers. Hum Reprod 12:101-105 (1997).
53. Rasmussen PE, Erb K, Westergaard LG. No evidence for decreasing semen quality in four birth cohorts of 1,055 Danish men born between 1950 and 1970. Fertil Steril 68:1059-1069 (1997).
54. Paulsen CA, Berman NG, Wang C. Data from men in greater Seattle area reveals no downward trend in semen quality: further evidence that deterioration of semen quality is not geographically uniform. Fertil Steril 65:1015-1020 (1996).
55. Van Waeleghem K, De Clercq N, Vermeulen L, Schoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod 11:325-329 (1996).
56. Zorn B, Virant-Klun I, Verdenik I, Meden-Vrtovec H. Semen quality changes among 2343 healthy Slovenian men included in an IVF-ET programme from 1983 to 1996. Int J Androl 22:178-183 (1999).
57. Gyllenborg J, Skakkebaek NE, Nielsen NC, Keiding N, Giwercman A. Secular and seasonal changes in semen quality among young Danish men: a statistical analysis of semen samples from 1927 donor candidates during 1977-1995. Int J Androl 22:28-36 (1999).
58. Andolz P, Bielsa MA, Vila J. Evolution of semen quality in North-eastern Spain: a study in 22,759 infertile men over a 36 year period. Hum Reprod 14:731-735 (1999).
59. Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril 65:1009-1014 (1996).
60. Saidi JA, Chang DT, Goluboff ET, Bagiella E, Olsen G, Fisch H. Declining sperm counts in the United States? A critical review. J Urol 161:460-462 (1999).
61. Auger J, Jouannet P. Evidence for regional differences of semen quality among fertile French men. Hum Reprod 12:740-745 (1997).
62. Zheng Y, Bonde JPE, Ernst E, Mortensen JT, Egense J. Is semen quality related to the year of birth among Danish infertility clients. Int J Epidemiol 26:1289-1297 (1997).
63. Younglai EV, Collins JA, Foster WG. Canadian semen quality: an analysis of sperm density among eleven academic fertility centers. Fertil Steril 70:76-80 (1998).
64. Grocock CA, Charlton HM, Pike MC. Role of the fetal pituitary in cryptorchidism induced by exogenous maternal oestrogen during pregnancy in mice. J Reprod Fertil 83:295-300 (1988).
65. Vorherr H, Messer RH, Vorherr UF, Jordan SW, Kornfeld M. Teratogenesis and carcinogenesis in rat offspring after transplacental and transmammary exposure to diethylstilbestrol. Biochem Pharmacol 28:1865-1877 (1979).
66. Weidner IS, Møller H, Jensen TK, Skakkebæk NE. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ Health Perspect 106:793-796 (1998).
67. Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect 107:297-302 (1999).
68. Moss AR, Osmond D, Bacchetti P, Torti FM, Gurgin V. Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol 124:39-52 (1986).
69. Schottenfeld D, Warshauer ME, Sherlock S, Zauber AG, Leder M, Payne R. The epidemiology of testicular cancer in young adults. Am J Epidemiol 112:232-246 (1980).
70. Osterlind A. Diverging trends in incidence and mortality of testicular cancer in Denmark, 1943-1982. Br J Cancer 53:501-505 (1986).
71. Bergstrom R, Adami HO, Mohner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Akre O, Hakulinen T. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J Natl Cancer Inst 88:727-733 (1996).
72. Depue RH, Pike MC, Henderson BE. Estrogen exposure during gestation and risk of testicular cancer. J Natl Cancer Inst 71:1151-1155 (1983).
73. Cocco P, Benichou J. Mortality from cancer of the male reproductive tract and environmental exposure to the anti-androgen p,p´-dichlorodiphenyldichloroethylene in the United States. Oncology 55:334-339 (1998).
74. Mocarelli P, Brambilla P, Gerthoux PM, Patterson DG Jr, Needham LL. Change in sex ratio with exposure to dioxin [Letter]. Lancet 348:409 (1996).
75. Potashnik G, Goldsmith J, Insler V. Dibromochloropropane-induced reduction of the sex-ratio in man. Andrologia 16:213-218 (1984).
76. De Cock J, Westveer K, Heederik D, Te Velde E, Van Kooij R. Time to pregnancy and occupational exposure to pesticides in fruit growers in The Netherlands. Occup Environ Med 52:429-430 (1994).
77. Marcus M, Kiely J, Xu F, McGeehin M, Jackson R, Sinks T. Changing sex ratio in the United States, 1969-1995. Fertil Steril 70:270-273 (1998).
78. Moller H. Change in male:female ratio among newborn infants in Denmark. Lancet 348:828-829 (1996).
79. Allan BB, Brant R, Seidel JE, Jarrell JF. Declining sex ratios in Canada. Can Med Assoc J 156:37-41 (1997).
80. Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries: a sentinel health indicator. JAMA 279:1018-1023 (1998).
81. Vartiainen T, Kartovaara L, Tuomisto J. Environmental chemicals and changes in sex ratio: analysis over 250 years in Finland. Environ Health Perspect 107:813-815 (1999).
82. James WH. Was the widespread decline in sex ratios at birth caused by reproductive hazards? Hum Reprod 13:1083-1084 (1998).
83. Akre O, Lipworth L, Tretli S, Linde A, Engstrand L, Adami HO, Melbye M, Andersen A, Ekbom A. Epstein-Barr virus and cytomegalovirus in relation to testicular-cancer risk: a nested case-control study. Int J Cancer 82:1-5 (1999).
84. Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med 332:1411-1416 (1995).
85. Hemminki E, Gissler M, Merilainen J. Reproductive effects of in utero exposure to estrogen and progestin drugs. Fertil Steril 71:1092-1098 (1998).
86. Falck F, Ricci A, Wolff MS, Godbold J, Deckers P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch Environ Health 47:143-146 (1992).
87. Wolff MS, Toniolo PG, Leel EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst 85:648-652 (1993).
88. Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect 101:372-377 (1993).
89. Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R. Effects of pesticides on the ratio of 16
90. Silinskas KC, Okey AB. Protection by 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) against mammary tumors and leukemia during prolonged feeding of 7,12-dimethylbenz(a)anthracene to female rats. J Natl Cancer Inst 55:653-657 (1975).
91. Scribner JD, Mottet NK. DDT acceleration of mammary gland tumors induced in the male Sprague-Dawley rat by 2-acetamidophenanthrene. Carcinogenesis 2:1235-1239 (1981).
92. Nesaretnam K, Hales E, Sohail M, Krausz T, Darbre P. 3,3´,4,4´-Tetrachlorobiphenyl (TCB) can enhance DMBA-induced mammary carcinogenesis in the rat. Eur J Cancer 34:389-393 (1998).
93. Ramamoorthy K, Gupta MS, Sun G, McDougal A, Safe SH. 3,3´,4,4´-Tetrachlorobiphenyl exhibits antiestrogenic and antitumorigenic activity in the rodent uterus and mammary and in human breast cancer cells. Carcinogenesis 20:115-123 (1999).
94. Kociba RJ, Keyes DG, Beger JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CL, Barnard SD, et al. Results of a 2-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in rats. Toxicol Appl Pharmacol 46:279-303 (1978).
95. Safe S. Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther 67:247-281 (1995).
96. Zacharewski T, Safe S. Antiestrogenic activity of TCDD and related compounds. In: Reproductive and Developmental Toxicology (Korach KS, ed). New York:Marcel Dekker, Inc., 1998;431-448.
97. López-Carrillo L, Blair A, López-Cervantes M, Cebrián M, Rueda C, Reyes R, Mohar A, Bravo J. Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: a case-control study from Mexico. Cancer Res 57:3728-3732 (1997).
98. van't Veer P, Lobbezoo IE, Martin-Moreno JM, Guallar E, Gomez-Aracena J, Kardinaal AFM, Kohlmeier L, Martin BC, Strain JJ, Thamm M, et al. DDT (dicophane) and postmenopausal breast cancer in Europe: case control study. Br J Med 315:81-85 (1997).
99. Hunter DJ, Hankinson SE, Laden F, Colditz G, Munson JE, Willett WC, Speizer FE, Wolff MS. Plasma organochlorine levels and the risk of breast cancer. New Engl J Med 337:1253-1258 (1997).
100. Schecter A, Toniolo P, Dai LC, Thuy LT, Wolff MS. Blood levels of DDT and breast cancer risk among women living in the north of Vietnam. Arch Environ Contam Toxicol 33:453-456 (1997).
101. Moysich KB, Ambrosone CB, Vena JE, Shields PG, Mendola P, Kostyniak P, Greizerstein H, Graham S, Marshall JR, Schisterman EF, et al. Environmental organochlorine exposure and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 7:181-188 (1998).
102. Hoyer AP, Grandjean P, Jorgensen T, Brock JW, Hartvig HB. Organochlorine exposure and risk of breast cancer. Lancet 352:1816-1820 (1998).
103. Guttes S, Failing K, Neumann K, Kleinstein J, Georgii S, Brunn H. Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease. Arch Environ Contam Toxicol 35:140-147 (1998).
104. Liljegren G, Hardell L, Lindstrom G, Dahl P, Magnuson A. Case-control study on breast cancer and adipose tissue concentrations of congener specific polychlorinated biphenyls, DDE and hexachlorobenzene. Eur J Cancer Prev 7:135-140 (1998).
105. Helzlsouer KJ, Alberg AJ, Huang HY, Hoffman SC, Strickland PT, Brock JW, Burse VW, Needham LL, Bell DA, Lavigne JA, et al. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol Biomarkers Prev 8:525-532 (1999).
106. Moysich KB, Shields PG, Freudenheim JL, Schisterman EF, Vena JE, Kostyniak P, Greizerstein H, Marshall JR, Graham S, Ambrosone CB. Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 8:41-44 (1999).
107. Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst 86:589-599 (1994).
108. Zheng T, Holford TR, Mayne ST, Tessari J, Owens PH, Zahm SH, Zhang B, Dubrow R, Ward B, Carter D, et al. Environmental exposure to hexachlorobenzene (HCB) and risk of female breast cancer in Connecticut. Cancer Epidemiol Biomarkers Prev 8:407-411 (1999).
109. Schneider J, Kinne D, Fracchia A, Pierce V, Anderson KE, Bradlow HL, Fishman J. Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci USA 79:3047-3051 (1982).
110. Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-Hydroxyestrone: the 'good' estrogen. J Endocrinol 150:S259-S263 (1996).
111. McDougal A, Wilson C, Safe S. Induction of estradiol 2-hydroxylase activity in MCF-7 human breast cancer cells by pesticides and carcinogens. Environ Toxicol Pharmacol 3:195-199 (1997).
112. McDougal A, Safe S. Induction of 16
113. Adlercreutz H, Gorbach SL, Goldin BR, Woods MN, Dwyer JT, Hämäläinen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst 86:1076-1082 (1994).
114. Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini-Hill A, Ross RK, Pike MC. A pilot study of urinary estrogen metabolites (16
115. Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini-Hill A, Ross RK, Pike MC. Urinary 2-hydroxyestrone/16
116. Gaido K, Dohme L, Wang F, Chen I, Blankvoort B, Ramamoorthy K, Safe S. Comparative estrogenic activity of organochlorine pesticide residues in food and wine extracts. Environ Health Perspect 106:1347-1351 (1998).
117. Bolger M. Unpublished data.
118. Verdeal K, Ryan DS. Naturally-occurring estrogens in plant foodstuffs - a review. J Food Prot 42:577-583 (1979).
119. Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 350:23-27 (1997).
120. Morton M, Arisaka O, Miyake A, Evans B. Analysis of phyto-oestrogens by gas chromatography-mass spectrometry. Environ Toxicol Pharmacol 7:221-225 (1999).
121. Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, White A, Kemeny M, Busch E, Nafziger AN. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiol Biomarkers Prev 7:489-496 (1998).
122. Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr 17:353-381 (1997).
123. Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 83:2223-2235 (1998).
124. Glassner B. The Culture of Fear. New York:Basic Books, 1999.
125. Gyllenborg J, Skakkebaek NE, Nielsen NC, Keiding N, Giwercman A. Secular and seasonal changes in semen quality among young Danish men: a statistical analysis of semen samples from 1927 donor candidates during 1977-1995. Int J Androl 22:28-36 (1999).
in a distinct manner from estradiol. Mol Cell Endocrinol 142:203-214 (1998).
/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect 103(suppl 7):147-150 (1995).
-/2-hydroxyestrone metabolite ratios in MCF-7 cells by pesticides, carcinogens, and antiestrogens does not predict mammary carcinogens. Environ Health Perspect 106:203-206 (1998).
-OHE1 and 2-OHE 1) in postmenopausal women with and without breast cancer. Environ Health Perspect 105(suppl 3):601-605 (1997).
-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 91:1067-1072 (1999).
Last Updated: April 11, 2000