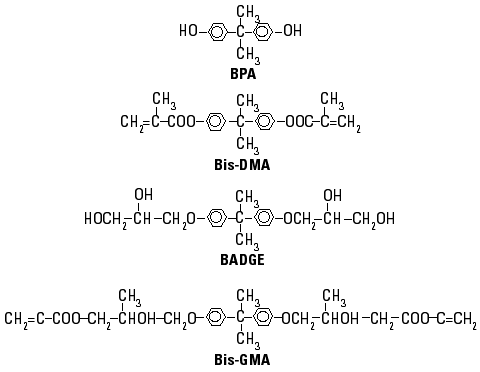
Figure 1. Chemical structures of bisphenol A and related compounds.
Rosa Pulgar,1 M. Fatima Olea-Serrano,2 Arancha Novillo-Fertrell,2 Ana Rivas,3 Patricia Pazos,3 Vicente Pedraza,3 Jose-Manuel Navajas,1 and Nicolas Olea3
1Department of Stomatology, School of Odontology; 2Department of Nutrition and Food Sciences, School of Pharmacy; 3Laboratory of Medical Investigations, Department of Radiology, School of Medicine; HUSC-University of Granada, Granada, Spain
http://ehpnet1.niehs.nih.gov/docs/2000/108p21-27pulgar/abstract.html
Address correspondence to N. Olea, Laboratory of Medical Investigations, Department of Radiology, University of Granada, 18071 Granada, Spain. Telephone: 34 58 24 2864. Fax: 34 58 24 9953. E-mail: nolea@goliat.ugr.esWe thank R. Davies for editorial assistance. A. Rivas and P. Pazos are on fellowships from the Spanish Ministry of Education and the Spanish Association Against Cancer, respectively.
This research was supported by grants from the Spanish Ministry of Health (FIS, 95/1959) and Education (CICYT, AMB97-1194-CE) and the Andalusian Regional Government, Department of Health (Consejería de Salud, JA, 231/97).
Received 25 March 1999; accepted 30 July 1999.
From a toxicologic standpoint, the migration of oligomers, monomers, and the precursors of synthetic polymers and other low-weight molecules from polymer networks must be carefully controlled because some of them can react with biologically important molecules. This is the case with BPA and bisphenol A diglycidylether (BADGE), which form adducts on DNA (7-9). BPA also binds to the estrogen receptor (10).
The estrogen receptor-mediated effects of BPA and related diphenyls make their detection an important issue. A study published in 1936 (11) demonstrated that diphenyl and diphenylmethanes had estrogenic activity in ovariectomized rats. Both compounds contained two hydroxyl groups in para positions. Later, Reid and Wilson (12) characterized further 4,4´-dihydroxy diphenylmethane-derived compounds with estrogenic activity, including molecular modifications of BPA with different hormonal activity. BPA mimics 17ß-estradiol in vitro (10,13-16) as well as in estrogen target organs in animal models (17-19). BPA mimicked natural and synthetic estrogens such as diethylstilbestrol in experimental models other than breast and uterine tissue (20-22). Besides BPA, its dimethacrylate derivative Bis-DMA (16,23,24), BADGE (16), and related diphenylalkanes (25) are estrogenic in different bioassays and systems. Bis-GMA increased uterine wet weight and uterine collagen content in ovariectomized mice (26) and induced cell proliferation on MCF-7 cells (27), although previous works were unable to show any proliferative effect of Bis-GMA except under extreme pH conditions (16).
BPA was detected in a culture medium for yeasts as an estrogenic contaminant that leached from polycarbonate bottles during autoclaving (10). Also, BPA and other aromatic monomers are contaminants in wine and mineral water stored in plastic containers (28,29), in microwave susceptors (30), in food from cans coated with epoxy resin lacquers (15), and in canned oily foods (31). More recently, we found that BPA and Bis-DMA were responsible for the estrogenicity of some commercial composites and sealants used in dentistry; they leached into the saliva of treated patients (16). These results have been confirmed by Arenholt-Bindslev et al. (32) and Fung et al. (33), who found BPA in saliva samples collected immediately after the placement of Delton LC (batch no. 940218; Dentsply, York, PA). A yeast-based bioassay confirmed the estrogenic activity of these saliva samples (32). In addition to BPA (16,32,33), Bis-DMA and Bis-GMA also leached from polymerized composites and sealants, and their presence among eluted compounds was demonstrated after in vitro polymerization (34-39). Leaching of components from composites and sealants may occur during the setting period of the resin and by the degradation of polymerized materials (40). Bis-DMA can also be a source of BPA because the enzymatic activity of saliva, esterases, extreme pH, and saliva storage can hydrolyze the dimethacrylate derivative (8,41). Figure 1 shows the chemical structure of BPA and related aromatic compounds.

Figure 1. Chemical structures of bisphenol A and related compounds.
The aim of the present study was to determine aromatic components eluted by in vitro polymerized Bis-GMA-based composites and sealants, to investigate how pH modifications affect the leaching of these components from the initial commercial product, and to assess their presence prior to polymerization. We found that BPA, Bis-DMA, BADGE, and Bis-GMA, among other aromatic components, leached from composites and sealants both before and after polymerization; their presence was always confirmed by gas chromatography/mass spectrometry (GC/MS).
Equipment. For HPLC we used a Konik model KNK-500 series A apparatus (Konik Instruments, Barcelona, Spain) connected with a Konik detector UVIS-200 and an HP integrator HP-3394A (Hewlett-Packard, Seville, Spain). For GC/MS we used a Fisons Carlo Erba 8000 chromatograph (Fisons Carlo Erba, Milan, Italy) and Fisons VG platform mass spectrometer.
Reagents. Acetonitrile (Panreac Química SA., Barcelona, Spain) was the HPLC reagent. Reference standards BPA (4,4´-isopropopylidenediphenol, FW 228.31), Bis-DMA (FW 364.44), bisphenol A etoxylate (EBPA; FW 316), and bisphenol A propoxylate (PBPA; FW 344) were purchased from Aldrich-Chemie (Albuch, Germany). BADGE (FW 340.45) was a gift from Gairesa, S.A. (La Coruña, Spain); Bis-DMA (FW 364.44) was obtained from NUPOL (Ivoclar, Liechtenstein). Analytical HCl, NaHCO3, and NaOH were purchased from E. Merck A.G. (Darmstadt, Germany).
Dental material. We used an Optilux VCL-300 polymerization lamp from Demetron Research Corporation (Danbury, CT). Its luminescence was tested and gave mean values of 435 mW/cm2. Polymerized composite samples were formed using 0.5-cm diameter glass cylinders 1 cm high so that all the samples presented the same geometric shape.
Composites and sealants. Dental materials tested in this study were selected from among commercial products available in the Spanish market. They were selected with the exclusive condition that the producer indicated that they were Bis-GMA-based resins. The composites and sealant studied are as follows:
GC/MS analysis. For the GC/MS analysis, we used a 15-m methyl silica capillary column (OV-P); an injector temperature of 240°C; and an ion source temperature of 200°C. The temperature gradient was as follows: initial temperature of 80°C (2 min); final temperature of 320°C; and rate of temperature increase of 10°C/min. The carrier gas was helium, the flow was 1.2 mL/min, and the injection volume was 0.2 µL.
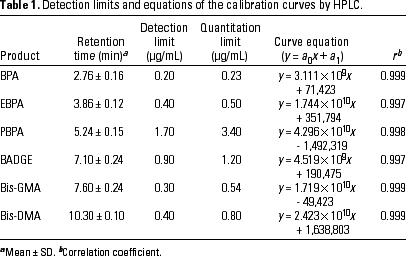
Qualitative analysis of BPA and related aromatic compounds. We analyzed solutions of reference standard products in ethanol by HPLC at concentrations between 10-7 and 10-3 mol/L. Mean retention times of the products are listed in Table 1. In the GC/MS chromatography, mean retention times ± SD (minutes) for each of the reference standards were 21.5 ± 0.2 for BPA; 22.8 ± 0.2 for Bis-GMA; 23.9 ± 0.1 for EBPA; 26.8 ± 0.2 for Bis-DMA; 27.7 ± 0.2 for PBPA, and 27.9 ± 0.3 for BADGE.
Dental methodology. Nonpolymerized samples were weighed (100 mg of each composite and 50 mg of the sealant), and samples were carefully decanted into the appropriate topaz glass vial to which 1 mL of distilled water was then added. A glass distillator and glass containers were used for water treatment and storage. After vigorous agitation, samples were left to settle for 24 hr.
Polymerized samples (100 mg) of each composite studied were compacted into a small glass cylinder to take on its geometric form. The cylinder was placed on a petri plate for compacting. The composite was polymerized to a 2-mm depth. Fifty milligrams of the sealant product was placed on a petri dish. Polymerization was immediately performed for 40 sec by situating the front of the lamp in close contact with the opposite side of the glass to that on which the cylinder was placed. Samples were extracted from the cylinder by applying pressure and were moved to a vial to continue the analysis. In all cases 1 mL distilled water was then added, and after vigorous agitation was left to settle for 24 hr.
The pH of the medium and the polymerized/nonpolymerized status were the paired variables. The pH values of 1, 7, 9, and 12 were regulated with 1 M HCl, distilled water, saturated solution of NaHCO3, and 1 M NaOH, respectively. The temperature used for each of these pH values was 37°C. Three samples of each commercial product were analyzed under these eight physicochemical conditions.
Quantitative analysis of BPA and related compounds. The detection and quantification limits were calculated in accordance with 10 concordant measurements of standard solutions for each of the products analyzed (BPA, EBPA, PBPA, BADGE, Bis-GMA, and Bis-DMA). Table 1 shows the detection limits that corresponded to the value 3 (42) deduced from experiments performed to establish the linearity of the detector. Peak areas were determined by using the integrator HP-3394. Calculations were made according to International Union of Pure and Applied Chemistry recommendations. Detection limits ranged from 0.20 µg/mL for BPA to 1.70 µg/mL for PBPA.
Calibration curve parameters and linearity of the detector responses are shown in Table 1 together with the estimated quantification limits. Standard solutions of variable concentration between 10-7 and 10-3 mol/L were prepared and 20 µL of each was injected into the HPLC. Calibration curves were constructed from 10 concordant measurements and were used for the subsequent quantification in the analyses.
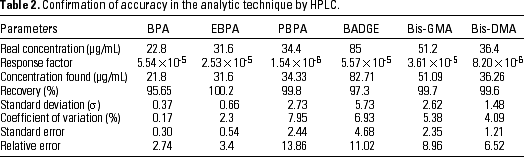
The accuracy of the chromatographic method was studied in the same conditions and was expressed as a percentage of recovery. The concentrations were calculated from the area of the peak and the mean response factor for each product (Table 2). Recovery percentages were always above 95% and ranged from 95.7% for BPA to 100.2% for EBPA.
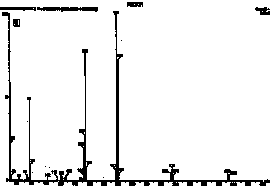
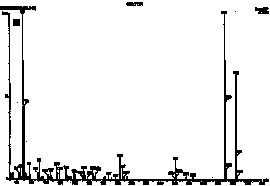
HPLC and GC/MS qualitative analysis. The compounds were first identified in HPLC from the retention times of each product. Figure 2 shows the chromatograms of polymerized Pekalux and Silux composites and the Delton sealant at pH 7. Peaks corresponding to the retention times of BPA, Bis-GMA, BADGE, PBPBA, EBPA, and Bis-DMA were observed. These findings were confirmed by GC/MS in all of the samples for which HPLC chromatograms showed the corresponding peaks. Figure 3 shows chromatograms (GC/MS) of compounds detected in the Delton sealant.
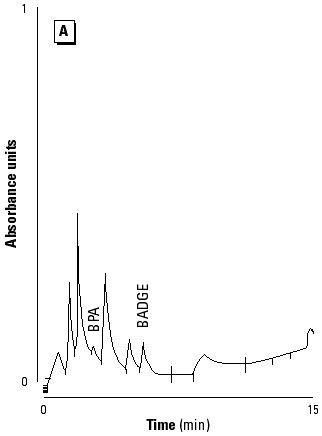
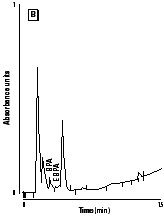

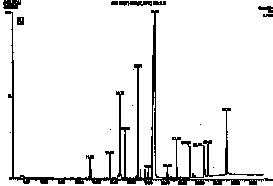
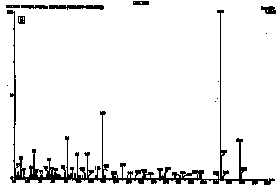
Figure 2. HPLC chromatograms of polymerized samples of (A) Pekalux (Bayer, Leverkusen, Germany) and (B) Silux (3M, St. Paul, MN) composites and (C) Delton (Dentsply, York, PA) sealant. Polymerized samples were left to settle in distilled water at pH 7, 371°C, for 24 hr. Leachable component corresponded to BPA, EBPA, PBPA, BADGE, Bis-GMA, and Bis-DMA.
|
|
|
|
Figure 3. Chromatograms by gas chromatography/mass spectrometry of a polymerized sample of the Delton (Dentsply, York, PA) sealant shown in Figure 2. (A) Peaks eluted at times 21.7, 22.8, and 27.0 min were identified as (B) BPA, (C) Bis-GMA, and (D) Bis-DMA, respectively.
HPLC quantitative analysis. To determine how pH modifications affected the leaching of components both from the initial nonpolymerized commercial product and from the in vitro polymerized product, 20-µL aliquots of the 1-mL water suspension in the different physicochemical conditions defined were analyzed by HPLC. Tables 3-10 list the mean ± SD (in micrograms per milliliter) of the concentrations of the aromatic components (BPA, EBPA, PBPA, BADGE, Bis-GMA, and Bis-DMA) quantified in each commercial sample before (nonpolymerized) and after polymerization (polymerized) in four pH conditions (acid, pH 1; neutral, pH 7; and alkaline, pH 9 and pH 12).
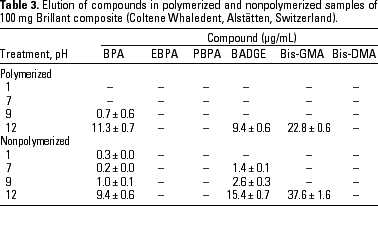
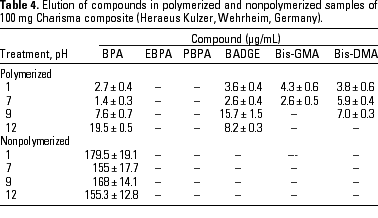
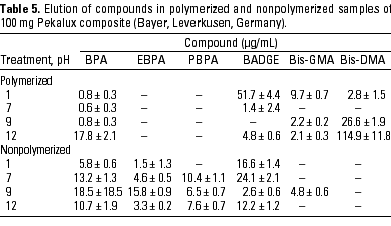
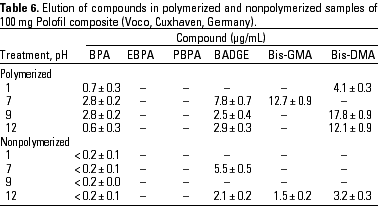
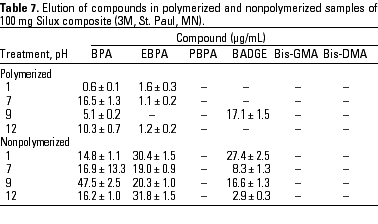
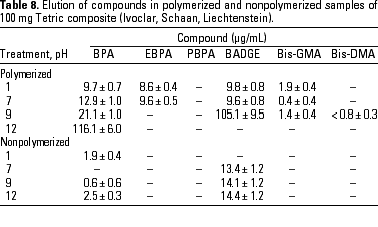
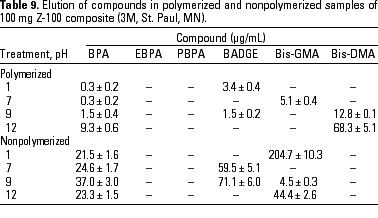
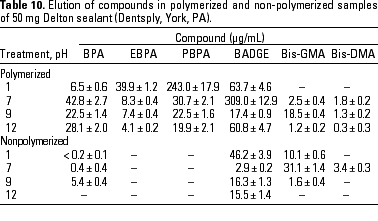
Data indicate the amount of compounds leached for a 24-hr period, expressed in micrograms per milliliter medium; because the volume of the sample was 1 mL, the data shown represent the total amount of leached components for 100 mg composite and 50 mg sealant. BPA was detected in all of the commercial samples studied both before and after polymerization. The maximal amount of BPA (1.8 µg/mg) was found in nonpolymerized Charisma independent of the pH. The lowest levels of BPA were found in nonpolymerized Polofil. In addition, polymerized Tetric and nonpolymerized Brillant were negative for this monomer at neutral pH. BADGE was found in all the samples (maximal 6.1 µg/mg in polymerized Delton) except nonpolymerized Charisma. The oligomer Bis-GMA was found in all polymerized and nonpolymerized samples except Silux samples, which were also negative for Bis-DMA and PBPA. However, Silux samples showed the presence of EBPA both before and after polymerization.
With respect to the presence of Bis-DMA, this monomer was found in all of the samples studied except the Brillant and Silux products. All others showed variable amounts of Bis-DMA (maximal 1.15 µg/mg for polymerized Pekalux at the highest alkaline pH) after polymerization (Charisma, Pekalux, Polofil, Tetric, Z-100, and Delton), and, in Polofil and Delton, also before polymerization. Finally, the presence of PBPA and EBPA was less frequent. PBPA was exclusively detected in nonpolymerized samples of Pekalux and in polymerized Delton. EBPA was detected in nonpolymerized Pekalux and Silux and in polymerized Delton, Silux, and Tetric.
The leaching of aromatic components from composites and sealants polymerized in vitro poses a clinical problem because of the demonstrated estrogenicity of some biphenolic compounds. We studied seven composites and one sealant and detected BPA in all of the commercial products analyzed, although some acid and neutral conditions were less conducive to its release. Regarding the release of other biphenolic compounds (25), all samples that showed detectable levels of Bis-DMA were also positive for the presence of Bis-GMA, BADGE, and BPA.
The elution of leachable compounds from samples greatly depends on the polymerization conditions (43), and standardization of these conditions was thus an essential first step in our study design. The polymerized and nonpolymerized resins were left undisturbed at rest and were submitted only to variable pH at a controlled temperature, so that the components migrated to the liquid phase without the use of organic solvents. Methanol (35,44,45) and water/ethanol combinations (46,47), a tonic drink (48), and food-simulating chemicals (47) have been reported in the literature as appropriate media for the extraction of polymerized composites. We used water in this study in an attempt to reproduce in vitro the conditions to which Bis-GMA-based resins are submitted when utilized in dentistry, as in our earlier work (16). Nevertheless, the use of water may result in lower levels of monomer elution than those found when organic solvents are applied, and this would account for quantitative differences between our findings and those of other authors.
The effect of pH variations on the leaching of monomers in dental microenvironments was reported previously (49). Extreme pH has some influence on the extraction of components from polymerized and nonpolymerized samples. In addition, leached components may be broken down in the stomach. The role of pH in the elution of compounds is evident in the results of the present study, especially for certain monomers in polymerized samples. For example, elution of BPA increased as the pH become more alkaline and was greater at pH 12 than at any other pH value. BPA values ranged between 0.014 (1.4 µg/mL) and 0.195 µg/mg (19.5 µg/mL) for the Charisma composite, between undetectable and 0.113 µg/mg (11.3 µg/mL) for Brillant, and between 0.129 (12.9 µg/mL) and 1.161 µg/mg (116.1 µg/mL) for the Tetric product, at pH 7 and 12, respectively. Although in the present study we highlight the data for BPA, these enhanced elution values at extreme pH were similar to those of other components found in most of the composites studied. Interestingly, when Bis-DMA was subjected to pH 11 for 30 min at 50°C, an almost 100% conversion of Bis-DMA to BPA was reported by Schmalz (41), who also showed the effect of saliva and esterase treatment on the hydrolysis of the Bis-DMA to BPA. Another recent study (50) confirmed that Bis-DMA was readily hydrolyzed to BPA when stored for 4 months in saliva at -20°C, whereas BPA was stable under the same storage conditions.
We stress that the composites and sealants are unstable and that to a greater or lesser degree, depending on the aggressiveness of the medium, it is always possible to detect the elution of monomers, olygomers, and precursors. These findings confirm reports that in vitro polymerization is not complete and that free monomers can be detected by different analytic methods (38,39,51,52). Several publications have appeared since our first report on BPA and Bis-DMA leaching from the Delton sealant (16) that may help to define a position regarding monomer leachability and their hormonal activity in vivo. For instance, Hamid and Hume (38) and Nathanson et al. (39) failed to find BPA leaching from several polymerized sealants, whereas Manabe et al. (53) found BPA leaching from unpolymerized dental materials and Arenholt-Bindslev et al. (32) and Fung et al. (33) found BPA in the saliva of patients treated with Delton.
Hamid and Hume (38) concluded that earlier concern about the possible adverse effects of BPA was questionable, whereas Nathanson et al. (39) claimed that there was no current need to use different sealants or restrict their use. Recently, we refuted point by point (54) the observations of Nathanson (39) and called for further research to address this issue. In the current study we used a reverse-phase HPLC method that substantially differs from the methods used by other authors. We set the wavelength of the detector at 280 nm, where aromatic molecules absorb and interference from other nonaromatic compounds is avoided. Wavelengths of 210-227 nm have been used previously for the determination of triethylene glycol dimethacrylate and Bis-GMA (34,38,39,55). BPA and the other compounds of interest to us (i.e., BADGE, Bis-DMA, and Bis-GMA) showed absorbance at 227 nm but also absorbed at 280 nm without interference from lineal ethylene glycol dimethacrylate derivatives, which had a spectrum with a single peak at 218 nm (48). Finally, chromatographic peaks must be confirmed by GC/MS and these techniques must be used correctly. Manabe et al. (53) pointed out that derivation with trimethylsilyl was necessary to detect BPA in dental material (53).
In fact, Bis-DMA was found by Nathanson et al. (39) in appreciable amounts (1.23 µg/mg in Delton and 0.39 µg/mg in Defender sealants, respectively), confirming previous reports of the presence of this compound leaching from composites and sealants (16,35,56). Bis-GMA was also found in the eluates of polymerized sealant samples by Hamid and Hume (38) and Nathanson et al. (39), as has been found for other Bis-GMA-based resins (16,35-37,48,56). Bis-DMA and Bis-GMA are two explanations for the estrogenicity of sealant and composite eluates. In addition to BPA (10,11,15-17), its dimethacrylate derivative Bis-DMA (16,23,24), the oligomer Bis-GMA (27), and other bisphenol A-related monomers (25,57) are all estrogenic. The cumulative estrogenic effect of these aromatic compounds should also be of concern.
With respect to the implications for patient care and dentist responsibility, more data must be gathered before a complacent attitude toward this hazard is adopted. As Söderholm and Mariotti (58) proposed, the main issue is to determine whether the estrogenic effects of dental sealant and composite monomers have any real clinical consequences. This concern should be addressed by new studies that focus on the toxicity--estrogenicity--of leached monomers, oligomers, and precursors, similar to the paper recently published by our group (25).
It will be difficult to give an a priori prediction of the risk to human health posed by such exposures, but new data may help assess this exposure within the general risks attributable to xenoestrogens and especially BPA (59). Four recent observations have raised concern about the estrogenicity of bisphenols. First, a more potent in vivo BPA effect has been demonstrated as compared to previous in vitro assays (19,21). For instance, 3 days of exposure to microgram levels of BPA (60-100 µg/rat/day) released from capsules promoted cellular proliferation in rat uterus and vagina, which showed molecular and morphologic alterations nearly identical to those induced by estradiol (60). The biologic significance of the 179 µg BPA eluted from a 100-mg polymerized sample of commercial composite in our study should be considered from this standpoint. Second, BPA seems to act on other target organs as well as the obvious organs [breast and uterus (21,22,49,61)]. Third, genetic differences in susceptibility to the estrogenic effect of BPA have raised concerns about subpopulations with a higher sensitivity to this estrogen (18,21). Fourth, bisphenol A is not the only molecule with proven in vitro estrogenicity that is used by the plastics industry (24,25,57).
The leaching of other components with estrogenic activity from polymerized composites and sealants cannot be ruled out because sparse information is available on their ingredients. For instance, commercial products use dibutylphthalate (35), dioctylphthalate (37), bis 2-ethylhexylphthalate, and dicyclohexylphthalate as catalyst components. Some of these phthalates are among the estrogenic chemicals described by Soto et al. (62) and Jobling et al. (63).
We confirm the leaching of BPA and other aromatic compounds from one sealant and we present new data on biphenolic monomers leaching from seven other commercial composites currently used in dentistry. To date, reports in the literature on the determination of products eluted from composites have considered other components. We present a study that centers on compounds with documented estrogenic activity.
References and Notes
1. Yalon M, Goldberg EP, Osborn D, Stacholy J, Sheets JW. Polycarbonate intraocular lenses. J Cataract Refract Surg 1449:393-395 (1988).
2. Vuillemin T, Raveh J, Stich H, Cottier H. Fixation of bone fragments with BIOCEM. First observations on humans. Arch Otolaryngol Head Neck Surg 113:836-840 (1987).
3. Kanawabe K, Tamura J, Yamamuro T, Nakamura T, Kokubo T, Yoshihara S. A new bioactive bone cement consisting of Bis-GMA resin and bioactive glass power. J Appl Biomat 4:135-141 (1995).
4. Simonsen RJ. Fissure sealants and the preventive resin restoration on the NHS. Br Dent J 6:238-239 (1988).
5. Manton DJ, Messer LB. Pit and fissure sealants: another major cornerstone in preventive dentistry. Aust Dent J 40:22-29 (1995).
6. Leinfelder KF. Posterior composite resins: the materials and their clinical performance. J Am Dent Assoc 126:663-676 (1995).
7. Steiner HR, Hönger B, Sagelsdorff P. Molecular dosimetry of DNA adducts in C3H mice treated with bisphenol-A diglycidyl ether. Carcinogenesis 13:969-972 (1992).
8. Atkinson A, Roy D. In vivo DNA adduct formation by bisphenol A. Environ Mol Mutagen 26:60-66 (1995).
9. Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Mizumi N, Yamaguchi F, Barrett JC. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syriam hamster embryo cells. Int J Cancer 75:290-294 (1998).
10. Krishnan AV, Starhis P, Permuth SF, Tokes L, Feldman D. Bisphenol A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132:2279-2286 (1993).
11. Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature 137:996 (1936).
12. Reid RE, Wilson E. The relation of estrogenic activity to structure in some 4,4´-dihydroxydiphenyl-methanes. J Am Chem Soc 66:967-968 (1944).
13. Gilbert J, Doré J-C, Bignon E, Pons M, Ojasoo T. Study of the effects of basic di- and tri-phenyl derivatives on malignant cell proliferation: an example of the application of correspondence factor analysis to structure-activity relationships (SAR). Quant Struct Act Relat 13:262-274 (1994).
14. Villalobos M, Olea N, Brotons JA, Olea-Serrano MF, Ruiz de Almodovar JM, Pedraza V. The E-Screen assay: comparison among different MCF7 cell stocks. Environ Health Perspect 103:844-850 (1995).
15. Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coating in food cans. Environ Health Perspect 103:608-612 (1995).
16. Olea N, Pulgar R, Pérez P, Olea-Serrano MF, Novillo-Fertrell A, Rivas A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect 104:298-305 (1996).
17. Ashby J, Tinwell H. Uterotrophic activity of bisphenol A in the immature rat. Environ Health Perspect 106:719-720 (1998).
18. Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect 105:70-76 (1997).
19. Colerange JB, Roy D. Profound effects of the weak environmental estrogen-like chemical bisphenol-A on the growth of the mammary gland of noble rats. J Steriod Biochem Mol Biol 60:153-160 (1997).
20. Dodge JA, Glasebrook AL, Magee DE, Phillips DL, Sato M, Short LL, Bryant HU. Environmental estrogens: effects on cholesterol lowering and bone in the ovariectomized rat. J Steroid Biochem Mol Biol 59:155-161 (1996).
21. Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol-A stimulates prolactin release in vitro and in vivo. Endocrinology 138:1780-1786 (1997).
22. Pfeiffer E, Rosenberg B, Deuschel S, Metzler M. Interference with microtubules and induction of micronuclei in vitro by various bisphenols. Mutat Res 390:21-31 (1997).
23. Schafer TE, Lapp CA, Hanes CM, Lewis JB, Wataha JC, Schuster GS. Estrogenicity of bisphenol-A and bisphenol-A dimethacrylate in vitro. J Biomed Mater Res 45:192-197 (1999).
24. Andersen HR, Andersson A-M, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect(suppl 1)107:89-108 (1999).
25. Perez P, Pulgar R, Olea-Serrano F, Villalobos M, Rivas A, Metzler M, Pedraza V, Olea N. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect 106:167-174 (1998).
26. Mariotti A, Söderholm KJ, Johnson S. The in vivo effects of BisGMA on murine uterine weight, nucleic-acids and collagen. Eur J Oral Sci 106:1022-1027 (1998).
27. Nathanson D, Ghulman M, Ashayeri N, Chou L. In vitro estrogenic activity of leachable components from dental sealants and composites [Abstract]. J Dent Res 78(special issue):130 (1999).
28. Larroque M, Vian L, Blaise A, Brun S. Methodes de dosage des monomeres residuels des resines epoxydiques dans des simulants du vine. J Chromatogr 445:107-117 (1988).
29. Lambert C, Larroque M. Chromatographic analysis of water and wine samples for phenolic compounds released from food-contact epoxy resins. J Chromatogr Sci 35:57-62 (1997).
30. Sharman M, Honeybone C, Jickels S, Castle L. Detection of residues of the epoxy adhesive component bisphenol A diglycidilether (BADGE) in microwave susceptors and its migration into food. Food Addit Contam 12:779-787 (1995).
31. Biedermann M, Bronz M, Grob K, Gfeller H, Schmid JP. BADGE and its accompanying compounds in canned oily foods: further results. Mirr Gebiere Lebensm Hyg 88:277-292 (1997).
32. Arenholt-Bindslev D, Breinholt V, Schmalz G, Preiss A. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants [Abstract]. J Dent Res 77(special issue B):692 (1998).
33. Fung YK, Ewoldsen NO, St Germain HA, Miaw C, Chou H-N, Gruninger SE, Eichmiller FC, Siew C. Pharmacokinetics of bisphenol-A from a dental sealant in humans [Abstract]. J Dent Res 78(special issue):270 (1999).
34. Ruyter IE, Svendsen SA. Remaining methacrylate groups in composite restorative materials. Acta Odontol Scand 36:75-82 (1977).
35. Vankerckhoven H, Lambrechts P, Van Beylen M, Vanherle G. Characterization of composite resins by NMR and TEM. J Dent Res 60:1957-1965 (1981).
36. Inoue K, Hayashi I. Residual monomer (BisGMA) of composite resins. J Oral Rehabil 9:493-497 (1982).
37. Rathbun, MA, Craig RG, Hanks CT, Filisko FE. Cytotoxicity of a BisGMA dental composite before and after leaching in organic solvents. J Biomed Mater Res 25:443-457 (1991).
38. Hamid A, Hume WR. A study of component release from resin pit and fissure sealants in vitro. Dent Mater 13:98-102 (1997).
39. Nathanson D, Lertpitayakun P, Lamkin MS, Mahnaz EB, Lee-Chou L. In vitro elution of leachable components from dental sealants. J Am Dent Assoc 128:1517-1523 (1997).
40. Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res 69:1450-1455 (1991).
41. Schmalz G, Preiss A, Arenholt-Bindslev D. Bisphenol-A content of resin monomers and degradation products [Abstract]. J Dent Res 77(special issue B):823 (1998).
42. MacDougall D, Amore FJ, Cox GV, Crosby DG. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal Chem 52:2242-2249 (1980).
43. Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N, Kawano J. High-performance liquid chromatographic estimation of eluates from denture base polymers. J Dent 17:84-89 (1989).
44. Taira M, Urabe H, Wakasa K, Yamaki M. The effects of the photo-initiator concentrations on the curing behaviors of bis-GMA monomer. Hiroshima Daigaku Shigaku Zasshi 21:382-386 (1989).
45. Spahl W, Budzikiewicz H. Qualitative analysis of dental resin composites by gas and liquid chromatography/mass spectrometry. Fresenius J Anal Chem 350:686-691 (1994).
46. Pham D, Ferracane JL. Leaching from light cured composites with variable degrees of conversion. J Dent Res 67: 903-908 (1988).
47. Ferracane JL. Elution of leachable components from composites. J Oral Rehabil 21:441-452 (1994).
48. Thompson LR, Miller EG, Bowles WH. Leaching of unpolymerized materials from orthodontic bonding resin. J Dent Res 61:989-992 (1982).
49. Dodds MWJ, Edgar WM. The relationship between plaque pH, plaque acid anion profiles, and oral carbohydrate retention after ingestion of several 'reference foods' by human subjects. J Dent Res 67:861-865 (1988).
50. Atkinson JC, Jones GK, Eichmiller F, Dickens S, Selwitz RH, Pillemer SR. In vitro measurements of salivary bisphenol-A, BisDMA and TEGDMA: A pilot study [Abstract]. J Dent Res 78(special issue):296 (1999).
51. Rueggeberg FA, Craig RG. Correlation of parameters used to estimate monomer conversion in a light cured composite. J Dent Res 67:932-937 (1988).
52. Shintani H. HPLC analysis of toxic additives and residual monomer from dental plate. J Liq Chromatogr 18:613-626 (1995).
53. Manabe A, Kaneko S, Numazawa S, Itoh K, Sasa R, Yoshida T, Inoue M, Hisamitsu H. Bisphenol-A leached out of dental materials [Abstract]. J Dent Res 78(special issue):296 (1999).
54. Olea N. Olea's response [Letter]. Environ Health Perspect 107:A290-292 (1999).
55. Munksgaard EC, Freund M. Enzymatic hydrolysis of (di) methacrylates and their polymers. Scan J Dent Res 98:261-266 (1990).
56. Ruyter IE, Oysted H. Composites for use in posterior teeth: composition and conversion. J Biomed Mater Res 21:11-23 (1987).
57. Olea-Serrano MF, Pulgar R, Pérez P, Metzler M, Olea N. Bisphenol-A: in vitro effects. In: Hormonally Active Agents in Food (Eisenbrand D, ed). New York:Wiley-VCH, 1998;161-180.
58. Söderholm KJ, Mariotti A. BisGMA based resins in dentistry: Are they safe? J Am Dent Assoc 130:201-209 (1999).
59. Feldman D. Estrogens from plastic--are we being exposed? [Editorial]. Endocrinology 138:1777-1779 (1997).
60. Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol-A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 139:2741-2747 (1998).
61. Ratnasabapathy R, Tom M, Post C. Modulation of the hepatic expression of the estrogen-regulated messenger-RNA stabilizing factor by estrogenic and antiestrogenic nonsteroidal xenobiotics. Biochem Pharmacol 53:1425-1434 (1997).
62. Soto AM, Sonnenschein C, Chung KL, Fernández MF, Olea N, Serrano FO. The E-SCREEN as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect 103(suppl 7):113-122 (1995).
63. Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalates plasticizers, are weakly estrogenic. Environ Health Perspect 103:582-587 (1995).
Last Updated: December 1, 1999